To drive future advancements in electronics, engineers must develop batteries that charge faster, store more energy, and last longer. Lithium-metal batteries (LMBs) stand out as a promising alternative to conventional lithium-ion (Li-ion) batteries, which currently dominate the market.
LMBs feature a lithium metal anode, unlike Li-ion batteries that rely on graphite or silicon-based anodes. This design allows for significantly higher energy densities. However, LMBs face challenges such as slow redox kinetics and poor cycling reversibility, which hinder charging speed and long-term efficiency.
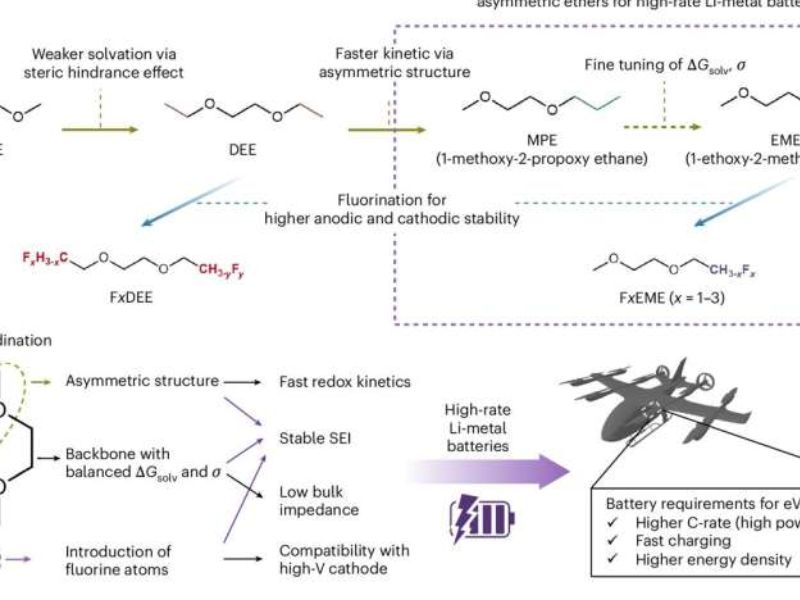
Researchers at Stanford University are tackling these limitations by developing new electrolyte solvents. Their recent study, published in Nature Energy, introduces asymmetric ether-based solvents that enhance both the charging speed and stability of LMBs.
“Our goal was to enable high-rate lithium-metal batteries by designing better solvent molecules,” said Rok Choi, the study’s first author. “Inspired by ethyl methyl carbonate (EMC), an asymmetric alkyl carbonate used in Li-ion batteries, we explored whether a similar asymmetric structure could improve ether solvents for LMBs.”
Traditional ether-based solvents, commonly used in battery electrolytes, contain symmetric molecular structures, which slow lithium-ion exchange and negatively impact charging speed and stability. To address this, Choi and his team investigated asymmetric ether solvents, which have molecules with different side groups.

Optimizing Solvents for Faster Lithium-Ion Transfer
“We designed solvents to minimize steric hindrance during Li+ desolvation,” Choi explained. “Symmetric solvents tend to block Li+ movement under an electric field, slowing charge transfer. In contrast, asymmetric solvents align in a way that enables faster Li+ reduction and desolvation.”
By optimizing dipole orientation—the alignment of positive and negative charges—the researchers improved charge transfer, enhanced lithium-ion movement, and promoted the formation of a stable solid-electrolyte interphase (SEI). This, in turn, helped create a uniform Li-plating layer on the anode.
“Our findings show that greater molecular asymmetry accelerates Li+ kinetics, leading to a more stable SEI and extended cycle life under high-rate conditions,” Choi said. “By optimizing both the ether backbone and fluorination degree, we developed F3EME as an ideal solvent, which demonstrated over 600 cycles in anode-free pouch cells—under test conditions simulating electric vertical take-off and landing (eVTOL) applications.”
Initial tests confirmed that asymmetric ether solvents significantly improved LMB performance and stability. Moving forward, Choi and his team plan to expand their research by designing similar electrolytes for other Li-based batteries, including Li-ion batteries with silicon anodes and Li-sulfur (Li-S) batteries.
“Building on this molecular design strategy, we aim to develop a broader range of solvents for various battery systems,” Choi added.
Read Original Article: TechXplore
Read More: Inception Launches From Stealth with a Groundbreaking AI Model