Blood Clotting Discovery Heralds A “New Era In Vascular Biology”

Scientists have uncovered a previously unrecognized biological process responsible for tissue and organ damage in low-oxygen conditions like heart attacks and strokes. The findings indicate that rupturing red blood cells, rather than blood clots, are to blame.
The microvasculature—a network of tiny blood vessels—is essential for supplying oxygen and nutrients to body tissues. When these vessels are damaged, it can lead to serious conditions such as heart attacks and strokes. In such cases, impaired microvascular function results in reduced blood flow, oxygen deprivation, tissue damage, and inflammation, all of which can make the condition more severe.
New Study Uncovers Red Blood Cells as Key Cause of Tissue Damage in Low-Oxygen Conditions
A recent study by researchers from institutions in Australia and New York has revealed a previously unidentified biological process responsible for tissue and organ damage in low-oxygen environments. Unlike earlier beliefs that blamed blood clots, this damage is actually caused by red blood cells.
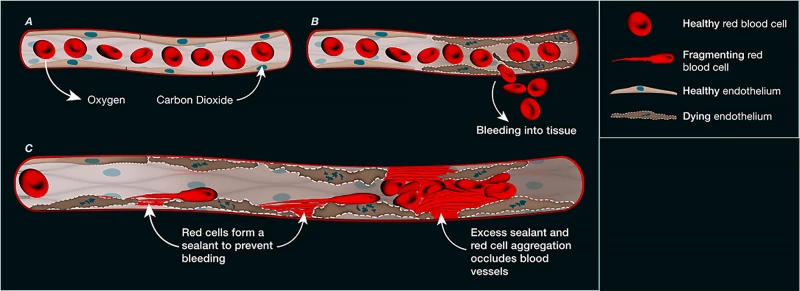
“We’ve uncovered an entirely new clotting mechanism that operates independently of the traditional system involving platelets and fibrin,” said Professor Shaun Jackson, lead author and founder of ThromBio, a company developing anti-clot therapies. “Instead, we found that dying cells trigger red blood cells to rupture, and their membranes act like biological glue—sealing damaged vessels and obstructing blood flow to vital organs.”
Traditionally, when a blood vessel is injured, platelets rapidly adhere to the site and to each other, forming a temporary plug. Simultaneously, a series of blood proteins activates fibrin, which forms a stabilizing mesh over the platelet plug, creating a durable clot to stop bleeding.
The researchers knew that both acute and long COVID could harm the body’s tiny blood vessels, leading to impaired circulation. Initially, the team suspected excessive fibrin was causing the problem, but blood thinners designed to break it down had limited success—prompting them to search for an alternative explanation.
ThromBio
Endothelial Damage Linked to Ruptured Red Blood Cells in COVID-19
In an analysis of over 1,000 blood vessels from deceased COVID-19 patients, researchers discovered significant damage to the endothelial cells—the cells lining the inside of blood vessels. This damage was widespread across small vessels in the lungs, heart, kidneys, and liver, with evidence that many of these endothelial cells had died. Under the microscope, the team observed deposits of a protein-like substance at the sites where cells had perished. Further investigation revealed that this material came from ruptured (hemolyzed) red blood cells, which had released their sticky contents, clogging the microvasculature.
Importantly, this newly identified type of microvascular blockage wasn’t exclusive to COVID-19. In mouse models, researchers observed the same process following heart attacks, strokes, and gut ischemia—a condition in which reduced blood flow deprives the intestines of oxygen and nutrients.
Jackson said the mechanism reveals why patients with severe COVID-19 or other critical illnesses often develop multi-organ failure, even when treatments control traditional clotting. “It marks an entirely new chapter in our understanding of vascular biology.”
The discovery carries clear implications for medical treatment. As noted, standard blood thinners (anticoagulants) are largely ineffective in treating microvascular complications in COVID-19, since traditional blood clots aren’t the primary issue.
“Instead of focusing on platelets or clots, future treatments could target the prevention of endothelial cell death or the subsequent damage to red blood cells,” Jackson explained. “By interrupting this process early, we may be able to maintain blood flow, safeguard organs, and ultimately save lives.”
Read the original article on: New Atlas
Read more: Innovative Device Analyzes Menstrual Blood for Health Indicators











Leave a Reply