Bi et al.
By applying a widely used culinary method, scientists have developed a foam incorporating carbon monoxide to enhance the efficacy of autophagy inhibitors. This experimental cancer therapy has shown varied results in clinical trials thus far. The innovative foam presents a hopeful avenue for advancing treatments across various cancer types.
Autophagy is an inherent process in which cells naturally break down and recycle impaired or malfunctioning intracellular elements to prevent their accumulation. Various factors like nutrient scarcity, oxidative stress, and the rapid growth of cancer cells can trigger this degradation pathway. In cancer cells, there is an elevation in autophagy, implying that suppressing this process might be an effective approach to treating the disease.
Investigating Ambiguous Outcomes
Medications that hinder autophagy, like the antimalarials chloroquine and hydroxychloroquine, have been employed in experimental cancer treatments to enhance the efficacy of chemotherapy. Nevertheless, outcomes from clinical trials have yielded ambiguous results, prompting investigators from the University of Iowa Carver College of Medicine to explore the reasons behind this inconsistency.
“Within those clinical trials, they found mixed results; there was some benefit, but for many patients, there was no benefit, which really pushed researchers back to the drawing board,” explained James Byrne, the study’s corresponding author.
In investigating why autophagy inhibition exhibited variable effectiveness, researchers stumbled upon an unexpected finding: in two trials, smokers appeared to respond more positively than non-smokers.
“When we looked at how the smokers did in those trials, we saw an increase in overall response in smokers that received the autophagy inhibitors, compared to (non-smoker) patients, and we also saw a pretty robust decrease in the target lesion size,” explained Byrne.
Unveiling the Role of Carbon Monoxide (CO)
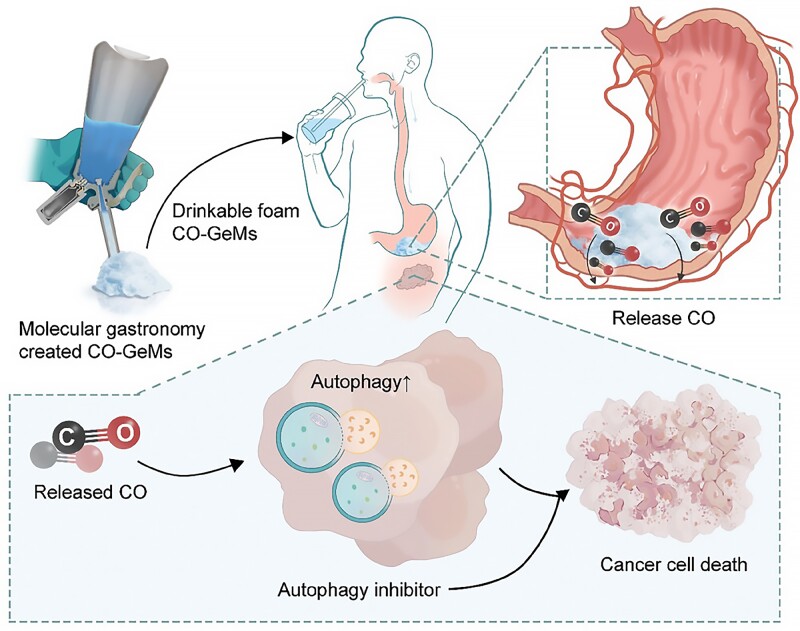
Earlier research had established that autophagy responds to gasotransmitters, a category of biological signaling molecules that includes carbon monoxide (CO), a component found in cigarette smoke. Consequently, the researchers focused on devising a method to administer exogenous CO.
“We are aware that smokers have elevated carbon monoxide levels, and while we strongly discourage smoking, this observation suggested that increased carbon monoxide might enhance the effectiveness of autophagy inhibitors,” noted Byrne. “Our goal is to harness this potential benefit and integrate it into a therapeutic platform.”
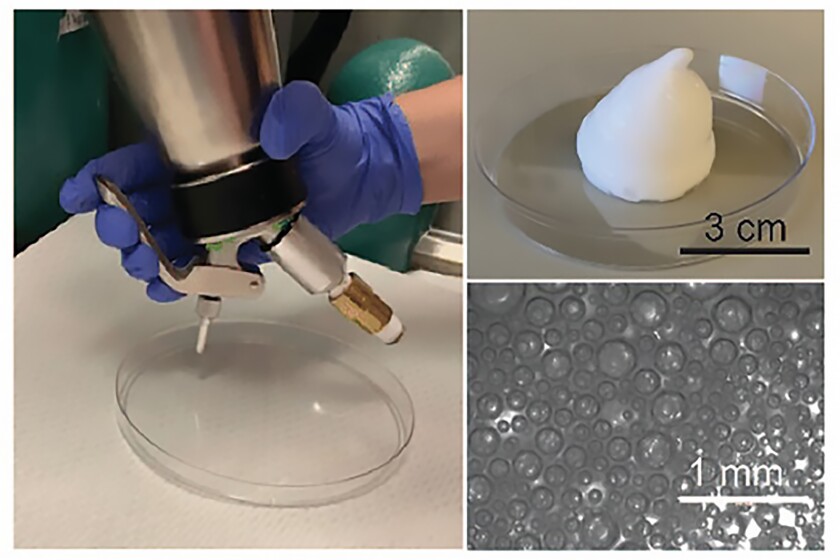
Remarkably, Byrne specializes in crafting gas-entrapping materials (GeMs), which include foams, gels, and solids made from safe, edible substances capable of being infused with various gas molecules. Drawing parallels to molecular gastronomy techniques often seen in cooking shows, the researchers utilized a whipping siphon to create a drinkable foam infused with carbon monoxide, termed a CO-GeM.
Synergistic Effects of Carbon Monoxide and Autophagy Inhibitors on Cancer Cells
In the laboratory, they initially tested the combined effect of carbon monoxide with autophagy inhibitors on human prostate, pancreatic, and lung cancer cells. Exposure to escalating doses of bafilomycin A1 (BAF-A1), chloroquine (CQ), and Lys05 revealed that the cytotoxicity (cell-damaging capacity) of the inhibitors increased in the presence of 250 ppm of carbon monoxide. Crucially, carbon monoxide did not impact cell viability in cancer cells and normal human intestinal cells in the absence of the autophagy inhibitor.
Bi et al.
Subsequently, the researchers assessed the effectiveness of their CO-GeM foam in mouse models with prostate and pancreatic cancer. The mice were categorized into four treatment groups: CO-GeM plus hydroxychloroquine (HCQ), CO-GeM alone, HCQ alone, and no treatment. Notably, mice receiving CO-GeM plus HCQ exhibited a substantial reduction in tumor growth within 21 days. Throughout the treatment, the mice maintained stable weights, and no indications of liver damage were observed, indicating the safety, biocompatibility, and tolerability of the combination treatment.
“The outcomes of this study affirm the concept that administering safe, therapeutic levels of CO, achievable through GeMs, can enhance the anti-cancer effects of autophagy inhibitors. This introduces a promising new approach that could enhance therapies for various cancers,” affirmed Byrne.
Delivery Methods and Safety Considerations
The applicability of the researchers’ discoveries to cancer treatments hinges on the delivery methods for carbon monoxide (CO) and the safety of the materials used. Previous studies utilizing inhaled CO have determined that CO treatment is highly safe, particularly for immunocompromised patients. Nevertheless, the researchers suggest that physicians may be more receptive to CO treatment if their innovative foams are employed instead of inhalation.
The researchers intend to further refine dosing formulations of CO-GeMs to ascertain doses that result in carboxyhemoglobin (COHb) levels – the standard measure for confirming the extent of CO exposure – falling below the FDA limit of 14%. Subsequently, they plan to evaluate the use of the novel foam in combination with autophagy inhibitors in human clinical trials.
Read the original article on: New Atlas
Read more: Improving AI Intuition in the Discovery of New Medicines
