In a paper released in Nature Communications, the research study team describes the complicated physical processes working to recognize the chemistry of ice formation. The molecular-level viewpoint of this process may assist in forecasting the formation and melting of ice, from singular crystals to glaciers and ice sheets. The latter is essential to measure environmental transformation connected with climate change and also global warming.
The team tracked down the primary step in ice development, called nucleation, which happens quickly, in a fraction of a billionth of a second when extremely mobile individual water molecules find each other and coalesce. However, conventional microscopes are too slow to follow the motion of water molecules, making it impossible to utilize them to monitor precisely how particles combine on top of solid surface areas.
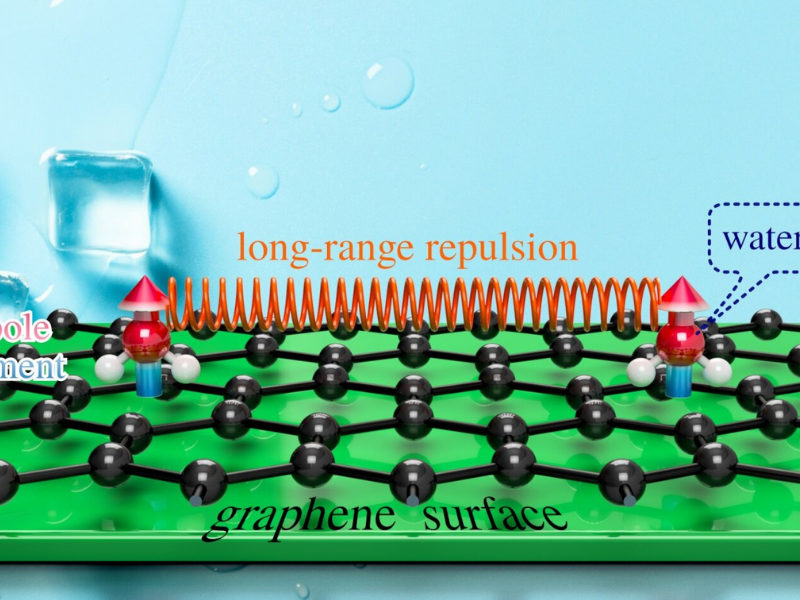
The research group used an avant-garde Helium Spin-Echo (HeSE) device to follow the atoms’ motion and molecules. The team utilized HeSE to study the water molecules’ motion on a model pristine graphene surface. The scientists made a notable finding: the water molecules repel each other and require enough energy to overcome said repulsion before ice can begin to form.
The combination of both experimental and academic approaches allowed the international team of scientists to decipher the behavior of the water molecules. For the first time, these have captured precisely how the initial step of ice formation at a surface area advances and enables them to suggest a previously unknown physical device.
Dr. Marco Sacchi, the co-author of the study and Royal Society University Research Fellow at the University of Surrey, claimed: “Our outcomes reveal that water molecules need to overcome a tiny but essential energy barrier before forming ice. We wish that our particular collaborative project will go some way to aiding us all comprehend the remarkable modifications that are taking place right across our planet.”
Dr. Anton Tamtögl, lead and a corresponding author from the Graz University of Technology, adds: “The observations entirely change our understanding of ice nucleation. The HeSE results looked extremely promising, but water motion was unbelievably complex and idicated counter-intuitive new physics. We decided that atomistic simulations were needed to decipher the results.”
Dr. Andrew Jardine, a reader in Experimental Physics from the University of Cambridge, one of the developers of the HeSE technique, stated: “The technique is entirely changing our ability to follow physical and chemical processes at the single-molecule level.”
Dr. Bill Allison from the University of Cambridge said: “Repulsion in between water molecules has not been taken into consideration during ice nucleation-this work will alter all that. The newly observed interactions likewise alter the rate at which nucleation occurs, and consequently, at which the ice can form. The work will therefore have important effects in preventing ice formation, which is relevant to fields as diverse as wind power, aviation as well as telecommunications.”
Originally published by the University of Surrey. Read the original article.
Reference: Anton Tamtögl et al, Motion of water monomers reveals a kinetic barrier to ice nucleation on graphene, Nature Communications (2021). DOI: 10.1038/s41467-021-23226-5