
A recent review has underscored how skin tone can influence the safety and effectiveness of certain medications and emphasized the need to reform current clinical drug trials to include more historically underrepresented populations.
Clinical trials play a crucial role in assessing the safety and efficacy of medications in humans. Although the primary aim of biomedical research is to enhance the health and well-being of the entire population, clinical trial participants often lack diversity. An analysis of 32,000 individuals involved in new drug trials in the U.S. in 2020 revealed that only 8% were Black, 6% Asian, 11% Hispanic, and 30% were aged 65 and older.
Impact of Skin Tone on Medication Effectiveness
A new review by Simon Groen, an assistant professor of evolutionary systems biology at the University of California, Riverside, and Sophie Zaaijer, a consultant and researcher at UC Riverside specializing in diversity, equity, and inclusion (DEI) in clinical trials, examined how skin tone—an aspect of race—affects medication effectiveness.
“Our review concludes that melanin, the pigment responsible for skin color, has a surprising affinity for certain drug compounds,” Groen stated. “The implications of melanin for drug safety and dosing have been largely neglected, raising serious concerns about the effectiveness of standard dosing, given the significant variation in skin tones among individuals.”
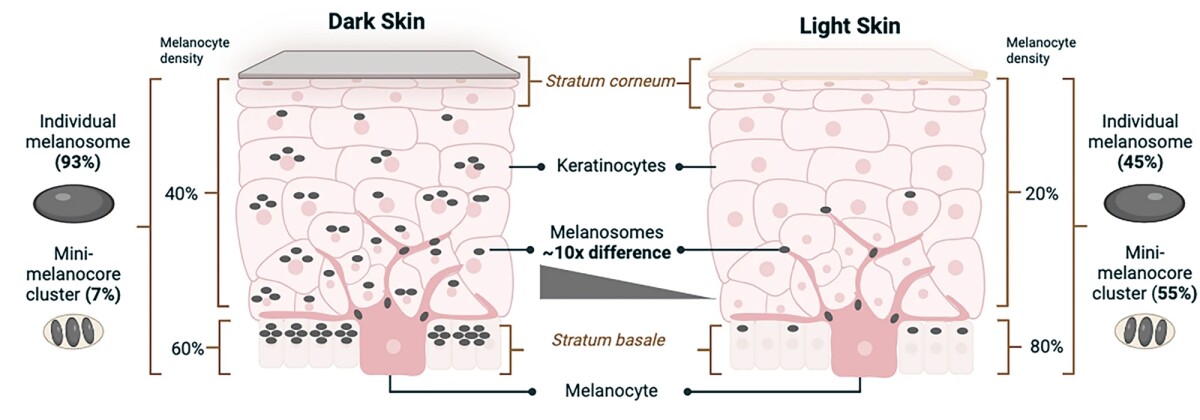
Zaaijer & Groen 2024
The key cells responsible for human skin tone are melanocytes, which produce melanin-containing melanosomes, and keratinocytes, which store these melanosomes. Variations in skin tone, ranging from light to dark, are determined by the number and characteristics of melanosomes within different skin layers. Dark skin contains a greater proportion of large individual melanosomes, while light skin has more clusters of smaller melanosomes. In light skin, melanosomes are primarily concentrated in the stratum basale, the deepest skin layer, whereas in dark skin, they are more diffusely distributed throughout the layers.
The Role of Melanin in Drug Interactions
A person’s unique combination of the two primary types of melanin—pheomelanin and eumelanin—determines their skin, hair, and eye color. Eumelanin, in particular, plays a significant role in drug interactions due to its chemical structure, which allows it to bind readily with various substances, including basic or neutrally charged drugs and metal ions. Compounds known to have an affinity for binding with eumelanin include cocaine, nicotine, the analgesic acetaminophen, and antibiotics like ampicillin, ciprofloxacin, and penicillin G, as well as antidepressants such as clomipramine and imipramine, and antipsychotic medications including chlorpromazine, clozapine, and haloperidol.

Depositphotos
Overlooked Interactions
Although the skin is the body’s largest organ, its interactions with eumelanin regarding drug pharmacokinetics (how the body processes a drug) and pharmacodynamics (the effects of a drug) have been largely ignored. Research shows that variations in skin eumelanin levels can influence nicotine use and dependence, particularly for individuals with darker skin using nicotine patches to quit smoking.
“Are we inadvertently disadvantaging smokers with darker skin tones if they rely on these patches?” Groen asked.
The researchers argue that current clinical trial guidelines inadequately address the impact of skin pigmentation on drug interactions.
“This oversight is concerning given the push for more diverse clinical trials, as noted in the FDA’s Diversity Action Plan,” Zaaijer said. “Yet, early-stage drug development still primarily tests on white populations of Northern European descent.”
Under the recently enacted Food and Drug Omnibus Reform Act (FDORA), the FDA requires sponsors of phase 3 clinical trials or other pivotal drug studies to submit a Diversity Action Plan to enhance enrollment from historically underrepresented populations. A “sponsor” can be an individual, pharmaceutical company, academic institution, or organization initiating a clinical investigation.
“The FDA recently published their draft guidelines,” Zaaijer said. “Once finalized in a few months, they will require the inclusion of patient diversity in clinical trials and preclinical R&D, along with guidance on pharmacokinetic variables to be tested for equitable drug access.”
Enhancing Diversity in Clinical Trials
Under the recently enacted Food and Drug Omnibus Reform Act (FDORA), the FDA requires sponsors of phase 3 clinical trials and other pivotal drug studies to submit a Diversity Action Plan to enhance enrollment from historically underrepresented populations. A “sponsor” can be an individual, pharmaceutical company, academic institution, or any organization responsible for initiating a clinical investigation.
“The FDA recently released draft guidelines,” Zaaijer noted. “Once finalized in a few months, they will mandate the inclusion of patient diversity in clinical trials and preclinical research, along with guidance on the pharmacokinetic variables to be evaluated in drug R&D for equitable access.”
Depositphotos
It’s a positive step, but real change will take time.
The Need for Diverse Human Cell Models in Drug Testing
“Drugs are often tested using a few human cell models primarily from Northern European donors,” Zaaijer noted. “Then they’re evaluated in rodent models. If successful, they move to clinical trials. But can we assume these drugs are safe for diverse populations if they haven’t been tested on human cell models from different ancestries? Would you bungee jump off a bridge knowing the ropes haven’t been tested for your weight? Probably not. So why is this acceptable for drugs?”
To address this, Groen and Zaaijer propose that pharmaceutical companies use 3D human skin models with varying pigmentation to assess the binding properties of new drugs across different skin tones.
“Skin pigmentation should be factored into safety and dosing assessments,” Zaaijer stated. “We are on the verge of a transformative era in the biomedical industry where inclusivity is essential, not optional.”
The researchers also encourage patients and clinical trial participants to ask questions such as, “Has this drug been tested for safety in people from various ancestral backgrounds, including mine?”
“If different ancestral backgrounds are considered in the early stages of drug discovery, diverse populations may have greater trust in the drug development process and be more likely to enroll in clinical trials, as they will be better informed about any potential risks,” Groen explained.
Read the original article on: New Atlas
Read more: Struggling to Focus? You Might Have Cognitive Disengagement Syndrome

